In recent years, Light Emitting Diodes, or LEDs, have revolutionized the illumination and display sectors, becoming ubiquitous in everyday life ranging from household lighting to large-scale outdoor displays. This article seeks to delve into the underlying principles by which LEDs emit light, providing a comprehensive overview that covers the physics, materials, and practical applications of this essential technology. auoky.com
## The Basics of Light Emission
At the core of the LED’s function lies quantum mechanics, particularly the processes of electron movement and energy band transitions within semiconductor materials. A fundamental grasp of these concepts can greatly illuminate the intricate nature of LED operation.
### 1. What is an LED?
An LED is a semiconductor device that emits light when an electric current passes through it. Unlike traditional incandescent or fluorescent bulbs which rely on thermal radiation or gas excitation, LEDs operate through electroluminescence, a process wherein a material emits light as a direct result of an electric current.
### 2. Semiconductors and Energy Bands
To fully understand LED technology, one must first comprehend the concept of semiconductors. Semiconductors are materials with electrical conductivity between that of conductors and insulators. In pure semiconductors, there are two bands of energy levels: the valence band and the conduction band.
– Valence Band: This is the energy band occupied by electrons in a normal state of the material.
– Conduction Band: This is the energy band where electrons can move freely, resulting in conduction of electricity.
The energy gap, known as the bandgap, between these two bands determines whether a semiconductor is a good conductor, insulator, or effective at emitting light.
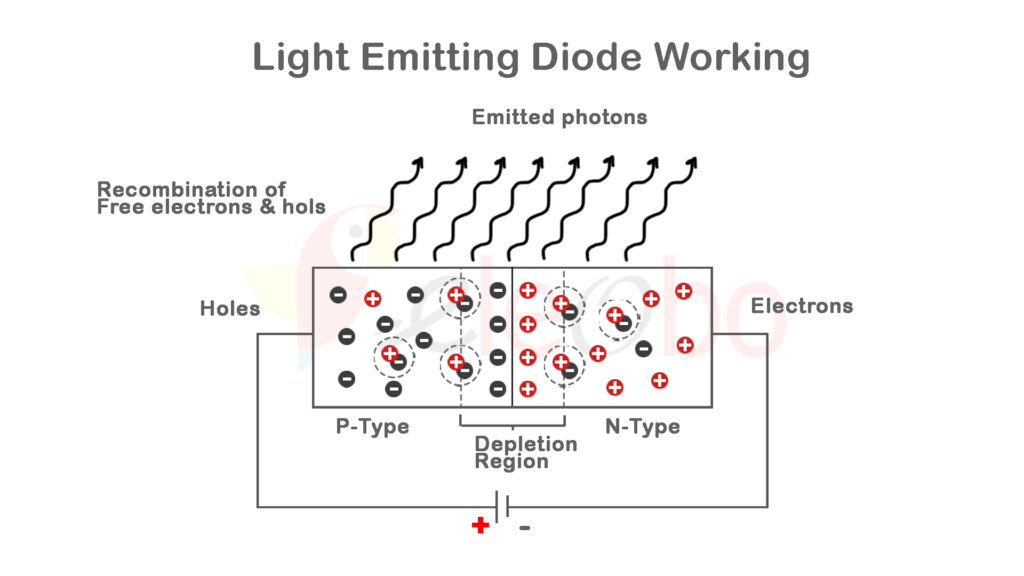
### 3. Creating an LED: Doping and P-N Junctions
To create an LED, a pure semiconductor (commonly gallium arsenide, gallium phosphide, or indium gallium nitride) undergoes a process called doping. Doping introduces impurities into the semiconductor material to control its electrical properties. This process creates two types of additional regions:
– N-type Semiconductor: Doping with elements that have more valence electrons (like phosphorus) introduces extra electrons, creating negatively charged carriers.
– P-type Semiconductor: Doping with elements that have fewer valence electrons (like boron) creates ‘holes’, or positively charged carriers.
When these two types of materials are joined, they form a P-N junction, which plays a crucial role in light emission.
### 4. Electron-Hole Recombination: The Heart of Light Emission
When a forward voltage is applied to the P-N junction, electrons from the N-type region are pushed towards the P-type region where holes exist. As these electrons meet holes, they recombine, effectively falling from the conduction band (higher energy state) to the valence band (lower energy state). During this process, the energy difference between these two states is released in the form of a photon, the basic unit of light.
The energy of the emitted photon, and therefore the color of the light generated, is determined by the specific bandgap energy of the semiconductor material used. For example:
– A larger energy gap typically produces blue or ultraviolet light, as seen in materials like gallium nitride.
– A smaller energy gap emits red or infrared light, common in materials such as gallium arsenide.
### 5. Materials and Their Properties
The choice of semiconductor material is vital in determining the efficiency, brightness, and color of the light emitted by an LED. Various compounds are utilized in the fabrication of LEDs, each selected for their specific properties:
– Gallium Nitride (GaN): Used primarily for blue and white LEDs, it has a wide bandgap and excellent thermal stability.
– Gallium Phosphide (GaP): Responsible for green and red light emissions, though it is less efficient than GaN in terms of light output.
– Aluminum Gallium Indium Phosphide (AlGaInP): Common for red, orange, and yellow LEDs, this alloy allows for a fine-tuned control over wavelength and brightness.
### 6. Efficiency and Advantages of LEDs
LEDs have proven to be highly efficient compared to traditional incandescent and fluorescent bulbs for several reasons:
– Energy Efficiency: LEDs convert a higher percentage of electricity into light rather than heat, making them more energy-efficient and resulting in lower power consumption.
– Longevity: The operational life of an LED can exceed 25,000 hours, dramatically surpassing that of incandescent bulbs (approximately 1,000 hours) and even fluorescents (around 10,000 hours).
– Durability: LEDs are solid-state devices, making them more resistant to impact and vibration, thus suitable for diverse environments and applications.
### 7. Applications of LED Technology
The applications of LED technology are vast and varied:
– General Lighting: From home and office lighting to streetlights, LEDs are becoming the preferred choice due to their efficiency and longevity.
– Display Technology: LEDs are used prominently in screens and displays ranging from small gadgets to large outdoor billboards due to their bright output and clarity.
– Automotive Lighting: Tail lights, headlights, and interior lighting in vehicles are increasingly using LEDs for enhanced visibility and energy savings.
### 8. Future of LED Technology
As technology progresses, advancements in LED technology are likely to continue. Research is underway to enhance color rendering, improve energy efficiency further, and include smart technology allowing for control via smartphones or home automation systems.
In conclusion, the ability of LEDs to emit light through the process of electroluminescence fundamentally alters how we think about lighting and displays. Their efficiency, longevity, and versatility have placed them at the forefront of modern illumination technology, ushering in a new era of sustainable and intelligent light sources. Understanding the science behind LED technology not only enhances appreciation for this technology but also encourages further innovation and application in various fields. auoky.com